I understand that the purpose of a salt bridge is to maintain the neutrality of the solutions in an electrochemical cell, but why do the ions being oxidized and reduced not pass through the salt bridge.
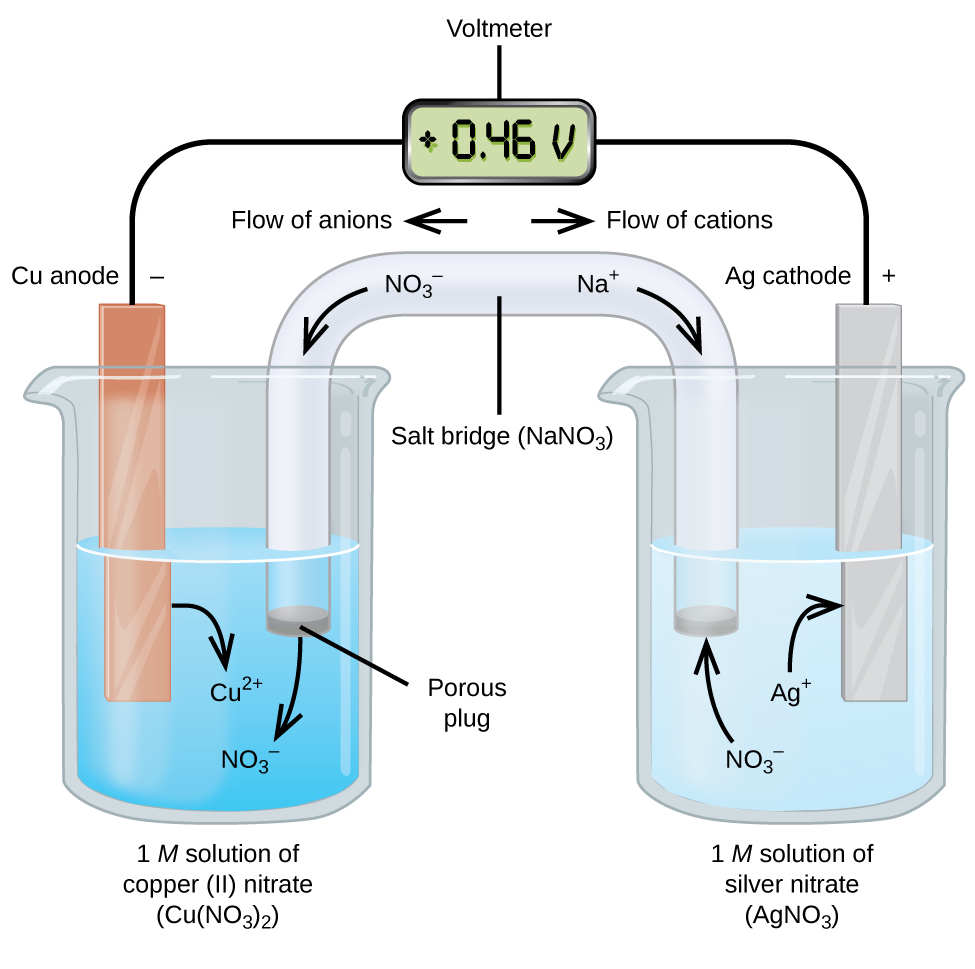
For example, in this electrochemical cell, why do the silver ions not pass through the salt bridge and become reduced in the copper sulfate solution. Wouldn't this cause less resistance in the circuit, or does the salt bridge prevent the metal ions from passing through?
No comments:
Post a Comment